Biomass Gasification
What is Gasification?
Gasification is technology developed to convert carbon based solid fuels to a gaseous form containing carbon monoxide and hydrogen. This gas is called the SynGas. Gaseous fuels are preferred to solid fuels due to its ease in handling and controlling flow and also better combustion performance. Biomass gasification is a world-wide accepted and a locally established technology for thermal energy and power generation applications. Biomass gasification is a renewable, low cost and environmentally friendly energy alternative.
Gasification Chemistry
Biomass consists of (on dry ash free basis):
- 50% Carbon
- 6% Hydrogen
- 44% Oxygen
Represented Chemical Formula: CH1.44O0.66
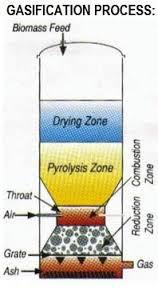
Pyrolysis Zone
Biomass is decomposed under a very low oxygen environment to form char and tar (and traces of methane, carbon monoxide, carbon dioxide and hydrogen).
C + heat → char + tar + CH4 + HCs
Combustion Zone
Heat for the pyrolysis reactions and reduction reactions is obtained when char, tar and biomass partially combusts with oxygen.
C + O2 → CO2 + heat
Reduction Zone
There are several intermediate reactions which contributes in forming the Producer Gas mixture. Composition of carbon monoxide, hydrogen and methane will depend on the rates of these intermediate reactions.
C + H2O + heat → CO + H2
C + 2H2 → CH4 + heat
3H2 + CO → CH4 + H2O + heat
H2O + CO → CO2 + H2 + heat
4H2 + CO2 → CH4 + 2H2O + heat
SynGas Properties
SynGas is a mixture of combustion gases synthesized by gasification of wood.
- Adiabatic Flame Temperature: 1100°C
- Heating Value / Calorific Value: 4.3 MJ/m3 at STP
- Gas Flow Rate: 2.5-3.0 m3/kg wood
- Dry Gas Composition (%)
- CO: 19 – 22
- CO2: 10 – 13
- N2: 50
- H2: 18 – 20
- CH4: 3
- Wet Gas Composition (%)
- H2O: 8 – 10
- CO: 12 – 17
- CO2: 13 – 15
- N2: 42 – 44
- H2: 18 – 19
- CH4: 2